
 As the vehicle battery is central to every circuit and system within the car, it is usually the first to check, regardless of where any auto-electrical problem appears.
As the vehicle battery is central to every circuit and system within the car, it is usually the first to check, regardless of where any auto-electrical problem appears.
In its original lead-acid format, the traditional battery has been around since the earliest days of motoring. In recent years the lead-acid battery has largely been phased out by the Nickel-iron alkaline powered battery.
Nickel-iron alkaline batteries offer superior performance to the lead-acid cell, although considerably more expensive, meaning that a surprisingly large number of classic car enthousiasts prefer to stand by the lead-acid battery, despite its quirks.
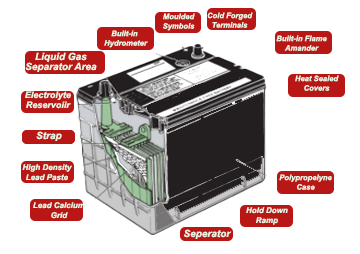
The 12V lead-acid battery is made up of a collection of cells, each producing about 2V.
 Within each cell is a collection of lead plates that are held in close proximity to each other. Between the plates is a solution of dilute sulphuric acid which acts as the electrolyte.
Within each cell is a collection of lead plates that are held in close proximity to each other. Between the plates is a solution of dilute sulphuric acid which acts as the electrolyte.
To ensure that the negative and positive plates do not touch, they are separated by thin sheets of either glass fibre or paper. This separating material is porous to allow free contact between the acid and the plates.
 In a battery of this type, the voltage is governed by the number of cells.
In a battery of this type, the voltage is governed by the number of cells.
The individual cells are joined in series by lead contact strips. If there is a breach between the cells, then the overall voltage of the battery will be significantly lowered. A 6V unit will contain three 2V cells, and a 12V unit will have six.

Overall battery capacity is governed by the surface area and number of plates. A larger battery will potentially contain a larger reserve of power and will generally be better suited to the heavier vehicle.
 As the car battery contains considerable quantities of sulphuric acid, the container that holds it needs to be strong, light, and resistant to chemical attack.
As the car battery contains considerable quantities of sulphuric acid, the container that holds it needs to be strong, light, and resistant to chemical attack.
Early batteries were produced using hard rubber, although modern batteries have cases made up of polypropylene plastic, which is lighter and will not break down with age.
The moulded casing is made in such a way as to isolate the cells and to hold the plates off the bottom to avoid any sediment, which might lead to a short circuit between plates.
Most current batteries feature vent holes to allow any gases to be safely expelled, especially when charging. The exception to this rule is the 'sealed for life' type battery containing calcium.
Purchasing a 'sealed for life' battery is not advised for classic cars as the likelihood of 'gassing' is higher if the charging voltage is not controlled to an extremely high degree.
Irrespective of which battery, it is essential to its health and longevity to be kept clean, as even a thin film of dirt may drain its power by leaching current across the terminals.

Furred, dirty, or poorly fitted terminals do not conduct current efficiently, leading to an under-charged battery. In a worst-case scenario, the terminals can overheat and ignite, causing severe damage to the engine if not the entire vehicle.
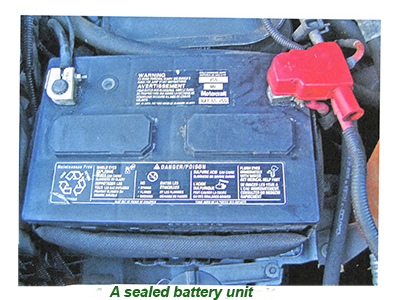 Maintaining a car battery requires very little in the way of technical skills, with terminals cleaned regularly with a wire brush if they are seen to be furred or dirty.
Maintaining a car battery requires very little in the way of technical skills, with terminals cleaned regularly with a wire brush if they are seen to be furred or dirty.
The condition of the cables should be the subject of regular scrutiny with particular attention paid to the earth point where the braided earth-strap meets the bodywork - this is a common point of corrosion and, therefore, resistance.
 Older lead batteries should be topped up with distilled water every one thousand miles, although newer "low maintenance" batteries only require to be topped up approximately once a year.
Older lead batteries should be topped up with distilled water every one thousand miles, although newer "low maintenance" batteries only require to be topped up approximately once a year.
 To get the best from a car battery, it is necessary to keep it charged, as repeated or prolonged discharge will shorten its working life. Most self-respecting classic car owners will have a battery charger in their possession, with newer models boasting an LED display. Fast charging, although convenient, is liable to reduce battery life.
To get the best from a car battery, it is necessary to keep it charged, as repeated or prolonged discharge will shorten its working life. Most self-respecting classic car owners will have a battery charger in their possession, with newer models boasting an LED display. Fast charging, although convenient, is liable to reduce battery life.